Bhor’s Atomic Model
Bohr’s atomic model is a classical model of the atom proposed by Danish physicist Niels Bohr in 1913. He proposed several postulates to explain the behavior of electrons in atoms. These postulates are as follows
Postulates of Bohr’s Model
Bhor’s Atomic Model
1. Stationary Orbits: Electrons orbit around the nucleus in specific, discrete energy levels or orbits, known as stationary orbits. These orbits are stable and do not result in the emission of energy.
2. Quantized Energy: The energy of an electron in a particular stationary orbit is quantized, meaning it can only have certain specific values.
The electron can absorb energy to move to a higher energy level or release energy to move to a lower energy level, with the energy difference between the levels corresponding to the emitted or absorbed radiation.
3. Angular Momentum: Bohr proposed that the angular momentum of an electron in a stationary orbit is quantized, meaning it can only have certain specific values.
The angular momentum of the electron is proportional to its orbital radius and velocity.
mvr = nh/2π
This equation expresses this quantization, where,
- m is the mass of the electron
- v is its velocity,
- r is the radius of the orbit, and
- h is Planck’s constant.
Here,
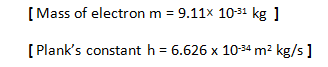
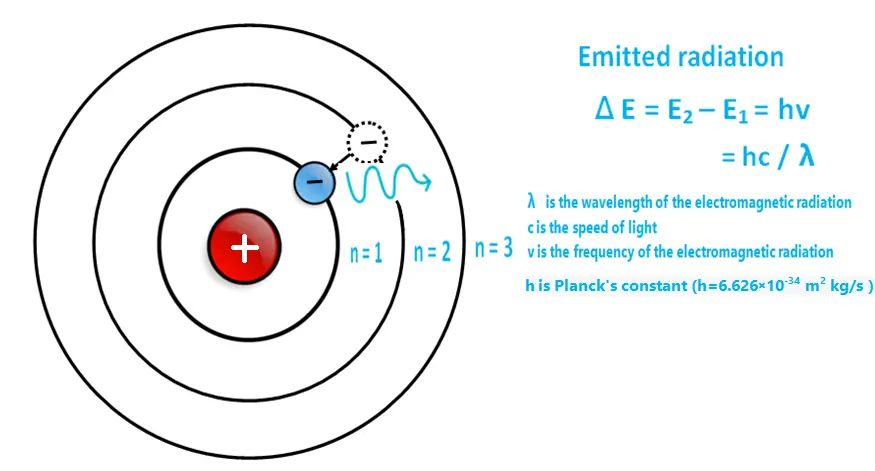
4. Electromagnetic Radiation: Bohr postulated that when an electron jumps from a higher energy level to a lower energy level, it emits the excess energy in the form of electromagnetic radiation or photons. The frequency of the emitted radiation is directly proportional to the energy difference between the two energy levels.
When an electron absorbs energy, it gets excited and jumps from lower energy level to a higher energy level or orbit.
The energy difference between two energy levels in an atom can be calculated using the following equation.
ΔE = E2 – E1 = h v
where:
- ΔE is the energy difference between the two energy levels
- E2 is the energy of the higher energy level
- E1 is the energy of the lower energy level
- h is Planck’s constant, which is a fundamental constant of nature that relates the energy of a photon to its frequency.
- v is the frequency of the electromagnetic radiation
5. Stability: Electrons in a stationary orbit do not emit energy and, therefore, do not spiral into the nucleus. They remain in stable orbits as long as they do not gain or lose energy.
These postulates of Bohr’s atomic model provided a framework for understanding the behavior of electrons in atoms.
Limitation of Bhor atomic model
The Bohr atomic model has several limitations. These limitations are given below.
1. This modal does not fully explain the behavior of electrons in atoms in case of more than one electron, such as multi-electron atoms or ions.
2. Bhor’s modal does not provide a detailed understanding of the electron’s exact path or trajectory around the nucleus. Fixed orbits for electrons are not consistent with the wave-like behavior of electrons described by quantum mechanics.
3. It does not explain the concept of electron spin, which is an important quantum mechanical property of electrons that affects their behavior and interactions with other particles.
4. It does not account for the concept of electron-electron repulsion, which plays a crucial role in the behavior of electrons in atoms with multiple electrons.
5. It does not explain phenomena such as chemical bonding, molecular structure, and other complex atomic interactions, which require a more advanced quantum mechanical model.
Bhor’s Atomic Model
Success of Niels Bohr’s Atomic Model
Niels Bohr’s atomic model was a significant success in the field of atomic physics during its time. The successes of Bohr’s atomic model are given below:
- Explanation of hydrogen spectrum:
Bohr’s model successfully explained the observed spectrum of hydrogen. The model provided a theoretical framework for understanding the discrete spectral lines observed in the hydrogen emission spectrum, which was not explained by earlier models.
- Quantization of energy levels:
Bohr’s model introduced the concept of quantized energy levels in atoms. This idea helped to explain the stability of atoms and the absence of continuous spectra, as observed in classical physics.
- Simplification of complex atomic structure:
Bohr’s model provided a simplified picture of the atomic structure and introduced the idea of electron orbits or shells, which provided a simple framework for describing the arrangement of electrons in atoms.
- Basis for further developments:
Bohr’s model laid the foundation for later models, such as the quantum mechanical model, which provided a more comprehensive and accurate description of atomic behavior.
Though Bhor’s model has been superseded by more advanced models, Bohr’s model still remains an important milestone in the history of the atomic model.
Related :
